Government mail service may be affected by the Canada Post labour disruption. Learn about how critical government mail will be handled.
About the indicator
- Particles and gases in the air, measured as positively or negatively charges ions, come down to the ground through rain, snow, or just by settling. This process is called atmospheric deposition.
- Alberta measures atmospheric deposition through the provincial, long-term Atmospheric Deposition Monitoring Program.
- Data from the monitoring program support Alberta’s Acid Deposition Management Framework, environmental impact assessments and national and international (Canada-United States) acid deposition science assessments.
- This indicator reports on concentrations of select ions and pH in precipitation (rain, hail or snow) from 2011 to 2020 across Alberta.
Atmospheric deposition facts
- Ions in precipitation come from both human and natural sources:
- Human influences include:
- electricity generation
- transportation and heating
- oil and gas processing
- agricultural activities
- Natural influences include:
- wind-blown dust
- wildfires
- lightning
- Human influences include:
- Atmospheric deposition:
- can affect aquatic and terrestrial ecosystems, human and wildlife health
- involves various ions that can either acidify or neutralize the environment, affecting soil chemistry and nutrient levels
Key ions in precipitation
- Atmospheric deposition includes both particles and gases that fall from the atmosphere to Earth's surface.
- Anions are negatively charged ions, such as sulphate (SO₄²⁻) and nitrate (NO₃⁻). These often come from air pollutants like nitrogen dioxide and sulphur dioxide.
- Cations are positively charged ions, including ammonium (NH₄⁺), calcium (Ca²⁺), magnesium (Mg²⁺), potassium (K⁺) and sodium (Na⁺).
- The cations Ca²⁺, Mg²⁺, K⁺ and Na⁺ are known as base cations because they can help neutralize acids.
- Rain and snow are naturally slightly acidic (pH around 5.6) because carbon dioxide in the air dissolves in water to form carbonic acid.
- Human-made pollutants can add more acidifying ions to precipitation, lowering the pH even further.
- Base cations from wind-blown dust can help neutralize this acidity.
- The mix of ions in precipitation varies by location, depending on:
- local and regional pollution sources
- wind patterns that carry polluted air
- land use (for example, urban vs. rural)
For example, acidifying ions are usually more concentrated near cities and industrial areas.
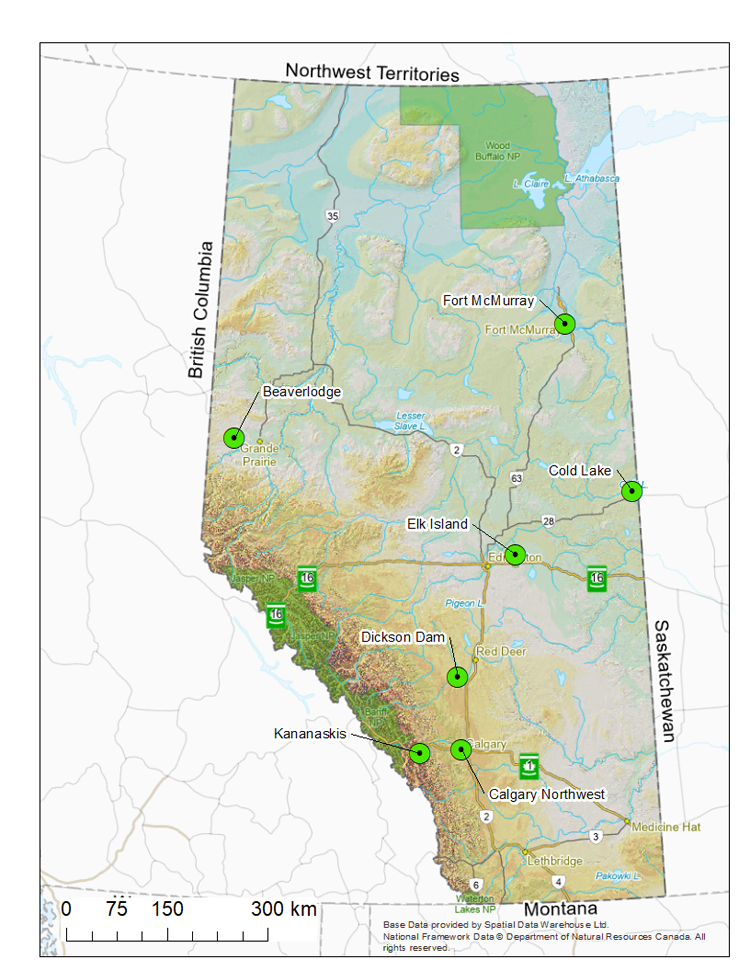
Figure 1. Alberta’s 7 atmospheric deposition monitoring program stations (green circles with black dot)
Methods
- For information on how the results for this indicator were calculated and for references, see: Condition of the Environment – Air Indicators
- Alberta collects atmospheric deposition data at 6 wet deposition stations and one total deposition station (wet and dry deposition) (Figure 1).
- These stations collect weekly, regionally representative samples to quantify deposition of pollutants and potential environmental impacts.
Summary of key results
Last updated: August 2025
- Between 2011 and 2020, NO₃⁻ and SO₄²⁻ ion concentrations in precipitation showed a statistically significant decreasing trend across the province. This aligns with decreasing trends reported for atmospheric nitrogen dioxide and sulphur dioxide in Alberta, implying a reduction in emissions of these gases is also affecting their atmospheric products.
- Precipitation pH showed a statistically significant increasing trend across Alberta between 2011 and 2022, meaning that wet deposition pH was less acidic.
- Ion concentrations at stations are affected by various sources across the province. Spatial variability appeared to be influenced by adjacent land use such as agricultural or industrial activities.
Variation across Alberta
- Some stations observed elevated mean concentrations for NH₄⁺. Agricultural land use and application of nitrogen fertilizer near Cold Lake, Dickson Dam and Elk Island could have impacted NH₄⁺ concentrations. Nitrogen fertilizer is a source of ammonia that reacts in the atmosphere to form NH₄⁺. Sources for elevated NH₄⁺ at the Calgary Northwest station are more complex and may reflect a variety of sources.
- Further variability in ion concentrations across Alberta is discussed in the report Atmospheric Deposition Monitoring Program: Concentrations and Trends: Selected Ions in Precipitation 2011-2020.
Changes over time
- Between 2011 and 2020, provincial trends for ion concentrations and pH in precipitation (Figure 3) varied.
- There was an overall decrease in provincial annual mean concentrations of NO₃⁻ and SO₄²⁻ in precipitation. These statistically significant trends are consistent with other related air indicators (for example, nitrogen dioxide and sulphur dioxide).
- Annual mean concentrations of NH₄⁺ and base cations appear to be increasing. However, the data currently available cannot determine statistically significant trends.
- A statistically significant increasing trend was observed for precipitation pH. The pH reported for Alberta around 2011 was typical for natural precipitation of 5.6, however, the increasing trend indicates precipitation is becoming less acidic relative to this natural value.
Figure 3. Box and whisker plots showing annual mean volume-weighted concentrations of ions and pH in precipitation
Lines crossing the plots indicate trends, with solid lines being statistically significant (p-value < 0.1) and dashed lines representing an insignificant trend. A box and whisker plot summarizes the overall range of a dataset. The box represents the middle 50% of the data with the horizontal line inside the box indicating the middle value of the dataset. Vertical lines extending from the top and bottom of the box show the overall spread of the data.
Seasonal variation
- Seasonal variability was observed for ion concentrations and pH in precipitation (Figure 4).
- NO₃⁻ concentrations were generally higher in winter and early spring, while SO₄²⁻ concentrations were higher in spring. These seasonal increases are likely associated with meteorological conditions supporting atmospheric oxidation of nitrogen dioxide and sulphur dioxide. In the case of NO₃⁻, lower temperatures could potentially promote its incorporation into particulate matter.
- Monthly average NH₄⁺ concentrations in precipitation were higher in spring (for example, April and May). This seasonality is likely associated with loss of snow cover and increased agricultural activities.
- Road salt, sanding and wind-blown dust likely contributed to higher base cation concentrations in winter and early spring.
- Precipitation pH was typically higher during spring, likely due to higher NH₄⁺ concentrations in precipitation during this period.
Figure 4. Box and whisker plots showing 2011-2020 monthly averaged volume-weighted concentrations of ions and pH in precipitation
Data access and limitations
- Precipitation chemistry data from the Atmospheric Deposition Monitoring Program is publicly available for 2013 forward from the National Atmospheric Database.
- Data collected prior to 2013 are available upon request (email: [email protected]).